Multilingual data support for clinical trials and global research studies.
Phase I – Patient Recruitment Support
Phase II & III – AE Narrative Translation
Phase III & IV – Data Entry & CRDM Support
Fozula Health Clinical Trial Support
Compliance with Good Clinical Practice (GCP)
🌍 Languages We Support in Clinical Trials


Multilingual Patient Recruitment & Communication
Assist with identifying, pre-screening, and enrolling participants across multiple languages
Provide interpretation or translation during the informed consent process (ICF)
Facilitate culturally sensitive patient communication to support retention and compliance
Support follow-up visits and calls with language-accurate messaging
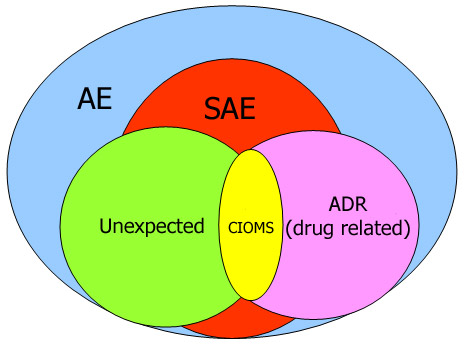




Clinical Trials Data Entry & Adverse Event (AE) Narrative Translation
Translate and verify AE narratives in compliance with study protocols and global safety reporting standards
Collect AE data during remote calls and input it into EDC platforms (e.g., Medidata, Oracle, Veeva)
Support narrative accuracy by reviewing and translating foreign-language responses into English
Assist in structured eCRF data entry and multilingual quality control in CRDM systems
🌐 🇩🇪 German
🌐🇫🇷 French
🌐 🇪🇸 Spanish
🌐 🇺🇸 English
🌐 UK English
🌐 🇨🇳 Chinese (Mandarin)
Monday – Sunday 24/7:
8:00 AM – 10:00 PM (EST)
8:00 AM – 10:00 AM (CET)
8:00 AM – 10:00 PM (CST)
Phone: +1 (213) 555-1234
Email: support@fozulahealth.com
Address:
FoZula Health
2400 W Sunset Blvd, Suite 567
Los Angeles, CA 90026
© 2024. All rights reserved.
Support for logistics and interpreter services may be available after hours by appointment.


